
Ions atomion #p 12 12 #e 12 10 atom ion #p 8 8 #e 8 10 Mg 2+ O 2-Ĭomplete Atomic Symbolcombines isotopes and ions Ions- Atoms which gain or lose an electron atom ion #pđ1đ1 #eđ1đ0 atom ion #pđ7đ7 #eđ7đ8 Why do the electrons change? Na1+ Cl1.Remember atoms are neutral, therefore if an atom has a charge we call it an ion.Atoms with a charge (positive or negative).Average mass of the atoms with all possible mass numbers.
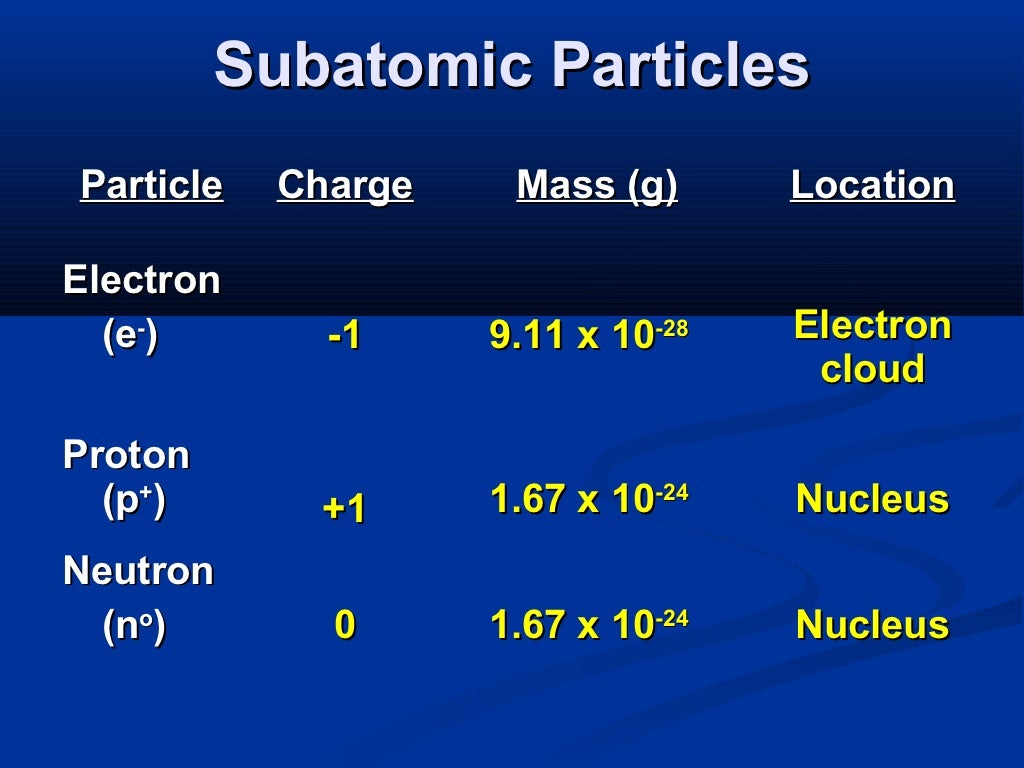
A given element can have atoms with several different mass numbers.What is the mass number of a calcium isotope with 19 neutrons? State the number of protons, neutrons, and electrons. Naturally occurring hydrogen consists of 3 isotopes, protium (H-1), deuterium (H-2), and tritium (H-3).Same number of protons, Different number of neutrons 37 17 Cl 35 17 Cl chlorine-35 chlorine-37.Most elements consist of several different isotopes.The atomic number identifies the element, but an element may have atoms with several different mass numbers.Mass number – Atomic number = # of neutrons O P Zn 8p+ 30p+.The total number of particles in the nucleus.Give the symbol of the element Mass Number Si 28 14 silicon-28 Atomic Numberĭetermine the Mass Numbers 4 Be 9.0122 27 Co 58.933 10 Ne 20.179 20 9 59.Show the mass number and atomic number.Determined by: the whole number closest to the Atomic Mass 14 Si Silicon 28.086 Atomic Mass.Counts the number of protons and neutrons in an atom OR.Number of Electrons 14 Si 14 electrons 14 protons -14 +14 = 0 neutral Number of protons = Number of electrons.State the number of protons for atoms of each of the following:.Counts the number of protons in an atom within the nucleus Atomic Number = # of protons 14 Si Silicon has 14 protons.Periodic Table 14 Si Silicon 28.086 Atomic Number Symbol Element name Atomic Mass B Groups: Transition and Inner Transition Metals.Periodic Table Categories: Metals Nonmetals Metalloids (B, Si, Ge, As, Sb, Te, At) Periodic Table Greatest Cheat Sheet Ever! Subatomic Particles How big is an atom? Imagine the nucleus is the size of a marble located in the middle of the football field, the electron would be in the classroom the size of a period! Farthest electron away from nucleus determines how atoms chemical to combine to one another.Spin around in set regions outside of nucleus.Responsible for chemical and physical properties.electrons electron proton nucleus neutron.What would atoms of an element, mixture and compound look like at a microscopic level?.Word origin: atom comes from the Greek word atomos meaning “ indivisible”

What is an atom? An atom is the smallest particle of an element that retains its identity in a chemical reaction.


 0 kommentar(er)
0 kommentar(er)
